生物大分子团队《EMBO Reports 》2022年
论文题目:Structural mechanism underpinning Thermus oshimai Pif1-mediated G-quadruplex unfolding
论文作者:Yang-Xue Dai#,Hai-Lei Guo#,Na-Nv Liu#,Wei-Fei Chen,Xia Ai,Hai-Hong Li,Bo Sun,Xi-Miao Hou*,Stephane Rety*,Xu-Guang Xi*
论文摘要:G-quadruplexes (G4s) are unusual stable DNA structures that cause genomic instability. To overcome the potential barriers formed by G4s, cells have evolved different families of proteins that unfold G4s. Pif1 is a DNA helicase from superfamily 1 (SF1) conserved from bacteria to humans with high G4-unwinding activity. Here, we present the first X-ray crystal structure of the Thermus oshimai Pif1 (ToPif1) complexed with a G4. Our structure reveals that ToPif1 recognizes the entire native G4 via a cluster of amino acids at domains 1B/2B which constitute a G4-Recognizing Surface (GRS). The overall structure of the G4 maintains its three-layered propeller-type G4 topology, without significant reorganization of G-tetrads upon protein binding. The three G-tetrads in G4 are recognized by GRS residues mainly through electrostatic, ionic interactions, and hydrogen bonds formed between the GRS residues and the ribose-phosphate backbone. Compared with previously solved structures of SF2 helicases in complex with G4, our structure reveals how helicases from distinct superfamilies adopt different strategies for recognizing and unfolding G4s.
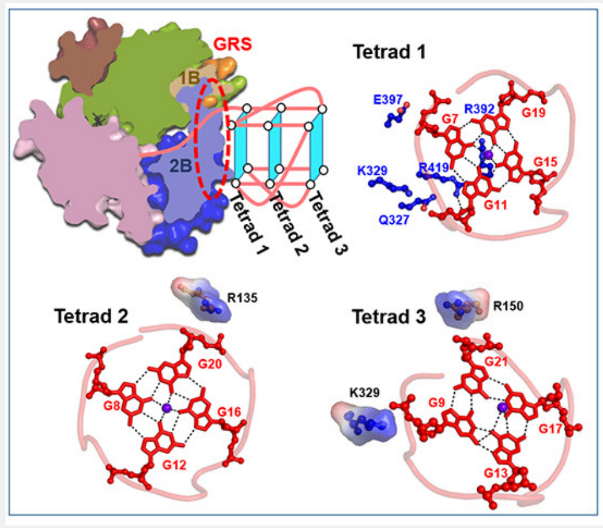
G-四链体(G4s)是一类异常稳定的DNA二级结构,会引起基因组不稳定。细胞为了克服G4形成的潜在障碍,已进化出不同的蛋白质家族来展开G4。其中,Pif1是一种来自超家族1 (SF1)的DNA解旋酶,从细菌到人类高度保守,能够有效展开G4。在这里,我们展示了第一个与G4 DNA复合的Thermus oshimai Pif1 (ToPif1) 晶体结构。我们的结构表明,1B/2B结构域上具有一个G4识别平面(GRS),ToPif1通过位于GRS上的一组氨基酸识别整个天然G4。G4在与ToPif1结合时保持其三层螺旋桨式的拓扑构型,没有显著的结构重组。G4中的三个G-四分体主要通过GRS残基和核糖-磷酸骨架之间形成的静电、离子相互作用和氢键被GRS残基识别。对比之前解析出的与G4-SF2 解旋酶复合体,我们的结构揭示了来自不同超家族的解旋酶如何采用不同的策略来识别和展开G4。
文章链接:https://www.embopress.org/doi/abs/10.15252/embr.202153874
